| Untriquadium | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 134Utq | |||||||||||||||||||
|
|||||||||||||||||||
| Appearance | |||||||||||||||||||
| unknown | |||||||||||||||||||
| General properties | |||||||||||||||||||
| Name, symbol, number | untriquadium, Utq, 134 | ||||||||||||||||||
| Pronunciation | /uːntraɪˈkwɒdiəm/ | ||||||||||||||||||
| Element category | superactinides | ||||||||||||||||||
| Group, period, block | N/A, 8, g | ||||||||||||||||||
| Mass number | not applicable[1] | ||||||||||||||||||
| Electron configuration | [Uuo] 5g86f48s28p2 (predicted)[2] 2, 8, 18, 32, 40, 22, 8, 4 (predicted)[2] 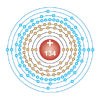
| ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| unknown | |||||||||||||||||||
| Atomic properties | |||||||||||||||||||
| unknown | |||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||
| Main article: Isotopes of untriquadium | |||||||||||||||||||
| |||||||||||||||||||
| v • t • e • r | |||||||||||||||||||
Untriquadium, Utq, is the temporary name for element 134. Isotopes are predicted within the bands 444Utq to 383Utq, 369Utq to 355Utq, and 317Utq to 313Utq. There may be isotopes in the band from the neutron dripline to 445Utq, but it is not possible to predict which ones are possible. Reported half-lives are all less than 1 hr, and most are under 1 sec. Fifty-three isotopes within the bands 444Utq to 426Utq and 423Utq to 389Utq are predicted to form. All Utq isotopes, predicted or guessed, will last less than 1000 sec after the event which led to their formation.
NUCLEAR PROPERTIES
INFORMATION SOURCES
While studies addressing specific issues have been carried out to very high N(1). and to moderate Z(2), (Z,N) or (Z,A) maps predicting half-lives and decay modes are almost completely limited to the region below Z = 130 and N = 220. There appears to be only one such map which extends beyond that region and is accessible(3).
(Z,N) maps for half-life and decay mode in Ref. 3 extend as high as Z = 175 and N = 333. Half-lives are reported as bands 3 orders of magnitude wide (0.001 - 1 sec, for example), and should be considered accurate only to within +/- orders of magnitude (presumably from band center. (A nuclide reported to be in the 0.001 - 1 sec band should be considered to have a possible half-life between 10^-4.5 sec and 10^1.5 sec.) Decay modes are limited alpha emission, beta emission, proton emission, and fission; and to the principal one for each nuclide. There are areas where two modes (or more) may be important, meaning that small uncertainties is model parameters could have produced different results. It is also possible that cluster decay may become important above the neutron shell closures at N = 228 and 308.
Ref. 3 does have two significant weakness in the way data are presented. Nuclides which are beta-stable are identified by black squares, overwriting decay mode and half-life information. In addition, nuclides having half-lives less than 10-09 sec are not reported, which obscures the distinction between nuclides having half-lives in the 10-09 and 10-14 sec band and nuclear drops whose half-life is under 10-14 sec.
Above Z around 126, predictions in Ref. 3 may not reach the neutron dripline. This can be an important limitation because the only processes which can form nuclei at more than atoms / star quantity generate very neutron-rich nuclei. It is possible to to make a crude, but conservative (high N) guess for the dripline's location by averaging predicted values for even-N nuclei.{See "The Final Element" (this wiki).} It is also possible to guess at regions of the (Z,N) or (Z,A) plane in which a fission barrier high enough to permit nuclides exists by using a first-order, liquid drop model. {See "Nuclear Guesswork" (this wiki).} Specific numbers are reported for these guesses, not with the expectation that they are accurate, but because they are consistent from element to element. They allow construction of a map which at least hints at where in the (Z,A) plane nuclides may be found.
GUESSED PROPERTIES
A simple liquid-drop picture indicates that 477Utq to 445Utq are unlikely to decay by neutron emission and are stable enough against fission to allow beta decay. Between 467Utq and 445Utq, Ref. 3 makes no predictions, but extrapolation from higher Z indicates that it would predict some short-lived, fission-decaying nuclides. Nuclear properties above 444Utq are highly uncertain, but it is likely that some relatively long-lived, beta-decaying isotopes of Utq are possible. It is possible to state that half-lives longer than 1 sec are implausible between the neutron dripline (nominally 477Utq) and 445Utq.
PREDICTED PROPERTIES
Isotopes in the band 444Utq - 430Utq are predicted to decay by beta emission. Since predicted half-lives are in the 10-06 - 0.001 sec range and beta decay partial half-lives far from stability have a minimum near 0.001 sec(4), that is about where half-lives should lie.
Isotopes in the band 429Utq - 425Utq are predicted to decay by fission. It appears to be possible for structure to destabilize a nuclide(5), so the data reported appear to be realistic, Half-lives are masked by other features of the map, but half-lives of nuclides of higher Z and comparable N indicate half-lives on the order of 10-06 to 0.001 sec. If this is the case, beta emission is an important secondary decay mode.
Isotopes in the band 424Utq - 403Utq are predicted to decay by beta emission, although a secondary fission branch is likely at the low-A end of the band. All half-lives are predicted to lie between 0.001 and 1 sec.
Most isotopes in the band 402Utq to 396Utq are predicted to decay principally by beta emission and have half-lives in the 0.001 - 1 sec range. Fission is predicted to dominate for 398Utq and 396Utq, and 396Utq is also predicted to have a half-life in the 10-06 - 0.001 sec range.
In the band 395Utq to 390Utq, most species are predicted to decay principally by beta emission and have half-lives in the 0.001 - 1 sec range. 394Utq and 391Utq, though are either nuclides with very short half-lives or drops too unstable to be nuclides. 391Utq is an odd-N nuclide.
There is a gap from 389Utq to 387Utq which probably contains very short-lived, fission decaying nuclides, but may be drops too unstable to be nuclides.
Between 386Utq and 384Utq, beta-decaying isotopes with 0.001 - 1 sec half-lives are predicted. This seems odd, but is not inconsistent with lower-Z nuclides with similar neutron counts.
383Utq is predicted to decay by fission with a half-life in the 10-09 - 10-06 range.
There is a gap from 382Utq to 370Utq in which properties are not reported. These may be short lived nuclides or nuclear drops whose half-life is less than 10-14 sec. It appears to be the expected destabilized region above N = 228.
Between 369Utq and 363Utq, all isotopes are predicted to decay by fission. Half-lives rise, as A declines, from <10-09 sec to the 0.001 - 1 sec range.
362Utq is predicted to decay by alpha emission with a half-life in the 1 - 1000 sec range. Neutron count for this isotope is 228.
In the band 361Utq to 355Utq, odd-N isotopes are predicted to decay by fission with half-lives under 0.001 sec. Even-N particles are either very short-lived nuclides or unstable nuclear drops.
There is a gap from 354Utq to 318Utq, in which particles are either nuclides with half-lives < 10-09 sec or nuclear drops too short-lived to qualify as nuclides. The latter possibility is more likely.
Odd-N particles in the band 317Utq - 313Utqare predicted to decay by fission with half-lives in the 10-09 - 10-06 sec range. Even-N particles are either very short-lived nuclides or unstable nuclear drops.
N = 258 CLOSURE
The model used to predict decay properties of Utq isotopes has a relatively weak neutron shell closure at N = 258. Some neutron-dripline studies have indicated a strong closure at N = 258. If that closure is strong, some isotopes in the 394Utq to 382Utq band may decay by beta emission rather than fission as predicted. They will probably be short-lived, but act as precursors to long-lived nuclides. By contrast, one or more isotopes in the band 381Utq to 370Utq may be long lived, with half-lives exceeding 1000 sec, because they are daughters of long-lived, alpha-decaying species. Interpolating between 472Uhq and 293Cn gives a reasonable value for maximum half-life of any nuclides stabilized by a strong N = 258 closure of 0.5 yr. Either alpha decay or beta decay may occur in this band, but fission can be expected to be suppressed.
These are not predictions of decay properties for nuclides in the vicinity of N = 258. This entire exercise is qualitative guesswork. No numbers, but a tantalizing hint of what might be.
OCCURRENCE
FORMATION
Where nuclear drops between the neutron dripline (nominally 477Utq) and 445Utq can be nuclides, they may form. Heavier isotopes may form directly from disintegrating neutron star material, and the remainder may form via beta decay chains from lower-Z nuclides. Since some of these chains may be terminated by short-lived, fission-decaying nuclides, it is not possible to say which isotopes of Utq in this range can form.
Nearly all nuclear drops in the bands 444Utq to 390Utq, 386Utq to 383Utq, 369Utq to 355Utq, and 317Utq to 313Utq are predicted to be nuclides. All are too far from the neutron dripline to form directly. It is possible to simulate the formation of nuclides via decay chains using data from Ref. 3 and assuming an initial distribution close to the neutron dripline. Details of the model are provided in "Nuclear Decay Chains at High A" in this wiki. Per that model, 53 predicted isotopes; 444Utq to 426Utq, 423Utq to 391Utq, and 389Utq; can form.
Neutron capture may be able to produce nuclides up to A around 360 before fission attrition stops further growth. It is implausible that neutron capture can form any Utq isotope. Fission infall may contribute small amounts of nuclides with A up to 406 (nominal).
PERSISTENCE
401Utq and heavier isotopes will vanish within 1000 sec after a neutron star merger which led to their formation, or lie at higher Z than beta-decay chains which end in nuclides which fission with a half-life not much greater than 1 sec.
400Utq to 364Utq lie at higher Z than the terminations of beta-decay chains that would populate them. In all cases, chains end in short-lived, fission-decaying nuclides.
363Utqto 353Utq lie at higher Z than the terminations of beta-decay chains that would populate them. Beta-decay chains end in long-lived nuclides (at lower Z), which decay by either fission or alpha emission.
If lighter isotopes of Utq can form, they are not expected to persist significantly.
Calculations done under maximum half-life assumptions and with all nuclides initially populated still point to all isotopes of Utq vanishing within 105.5 (3.16E05) sec.
N = 258 SHELL CLOSURE
Some studies of the neutron dripline indicate a strong shell closure at N = 258, instead of the relatively weak one occurring in the predictive models, If so, and if peak half-lives in the region do approach 0.5 yr; it is possible that one or more Utq isotopes may persist for up to 60 yrs, probably as a daughter of a long-lived ancestor.
ATOMIC PROPERTIES
Utq is expected to be an 8th period active metal (superactinide). Its consensus electron configuration has been predicted(6) to be [Og] 5g8 6f4 8s2 8p21/2.
REFERENCES
1. for example, "Nuclear Energy Density Functionals: What Do We Really Know?"; Aurel Bulgac, Michael McNeil Forbes, and Shi Jin; Researchgate publication 279633220 or arXiv: 1506.09195v1 [nucl-th] 30 Jun 2015.
2. for example "Fission Mechanism of Exotic Nuclei"; Research Group for Heavy Element Nuclear Science; http://asrc.jaea.go.jp/soshiki/gr/HENS-gr/np/research/pageFission_e.html.; 17 Sept 17.
3. "Decay Modes and a Limit of Existence of Nuclei"; H. Koura; 4th Int. Conf. on the Chemistry and Physics of Transactinide Elements; Sept. 2011.
4. "Nuclear Properties for Astrophysical Applications"; P. Moller & J. R. Nix; Los Alamos National Laboratory website; search by "LANL, T2", then "Nuclear Properties for Astrophysical Applications".
5. "Magic Numbers of Ultraheavy Nuclei"; Vitali Denisov; Physics of Atomic Nuclei; researchgate.net/publications/225734594; July 2005.
6. "Extended Periodic Table", Wikipedia.
7. Other references are found in the wiki articles cited.
| 9-Period Periodic Table of Elements | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | 1 H |
2 He | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 55 Cs |
56 Ba |
57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | 87 Fr |
88 Ra |
89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | 119 Uue |
120 Ubn |
121 Ubu |
122 Ubb |
123 Ubt |
124 Ubq |
125 Ubp |
126 Ubh |
127 Ubs |
128 Ubo |
129 Ube |
130 Utn |
131 Utu |
132 Utb |
133 Utt |
134 Utq |
135 Utp |
136 Uth |
137 Uts |
138 Uto |
139 Ute |
140 Uqn |
141 Uqu |
142 Uqb |
143 Uqt |
144 Uqq |
145 Uqp |
146 Uqh |
147 Uqs |
148 Uqo |
149 Uqe |
150 Upn |
151 Upu |
152 Upb |
153 Upt |
154 Upq |
155 Upp |
156 Uph |
157 Ups |
158 Upo |
159 Upe |
160 Uhn |
161 Uhu |
162 Uhb |
163 Uht |
164 Uhq |
165 Uhp |
166 Uhh |
167 Uhs |
168 Uho |
169 Uhe |
170 Usn |
171 Usu |
172 Usb | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | 173 Ust |
174 Usq | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(07-04-20)
